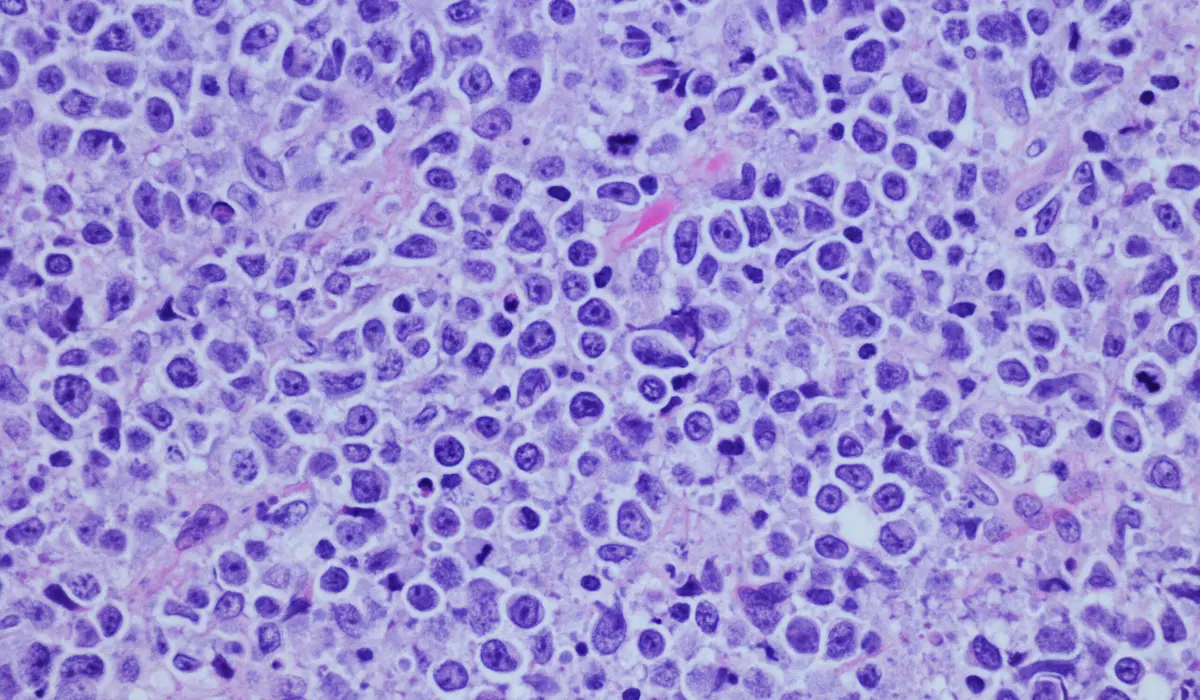
4SC AG, a biotech company specializing in treating advanced-stage cutaneous T-cell lymphoma (CTCL), has reached a significant milestone in the UK for its oral maintenance therapy, resminostat. The company received a Paediatric Investigation Plan (PIP) waiver from the UK’s Medicines & Healthcare products Regulatory Agency (MHRA), removing the need to conduct clinical studies in children. This development advances 4SC’s path toward a Marketing Authorisation Application for resminostat in the UK.
In the regulatory landscape, pharmaceutical companies are often required to submit a PIP, detailing plans for testing new medicines on pediatric populations. However, waivers are granted in cases where pediatric studies aren’t necessary or suitable, such as with resminostat. This drug is designed for advanced-stage CTCL, a rare cancer that primarily affects older adults.
Jason Loveridge, Ph.D., CEO of 4SC, emphasized the significance of this waiver, as it enables the company to move forward with their marketing application while saving time and resources that would have been required for a pediatric trial. It allows 4SC to focus more keenly on getting resminostat into the hands of adult patients with CTCL.
In addition to the UK application, 4SC submitted a Marketing Authorisation Application to the European Medicines Agency (EMA) in February 2024 for CTCL treatment in Europe. Applications for the UK and Switzerland are in preparation. Simultaneously, the company submitted a pre-New Drug Application (NDA) meeting request to the U.S. Food and Drug Administration (FDA) earlier this year.
4SC’s swift progress in seeking regulatory approvals demonstrates their commitment to improving the lives of patients suffering from CTCL. With the PIP waiver simplifying their UK approval process, the company is better positioned to bring this treatment to those in need while focusing on expanding its availability across Europe and beyond.